SS-31
From Peptidepedia, the trusted peptide wiki.


- SS-31 is a small peptide that helps mitochondria (cell powerhouses) produce more energy efficiently.
- It works by protecting a special fat called cardiolipin inside mitochondria from damage.
- Studies show it may help people with heart problems and muscle weakness, though more research is needed.
SS-31 (Elamipretide) is a mitochondria-targeted tetrapeptide that has gained significant attention for its potential to improve cellular energy production, reduce oxidative stress, and support cardiovascular, renal, and neuromuscular function. Popular among biohackers, longevity enthusiasts, and individuals with mitochondrial dysfunction, SS-31 is typically administered subcutaneously at doses ranging from 10–40 mg daily, with research protocols often spanning 4–12 weeks depending on the condition being addressed.
What Is SS-31?
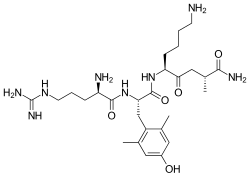
SS-31, also known as Elamipretide, Bendavia, or MTP-131, is a synthetic aromatic-cationic tetrapeptide with the amino acid sequence D-Arg-Dmt-Lys-Phe-NH2 (where Dmt represents 2',6'-dimethyltyrosine). Developed by Stealth BioTherapeutics, this small peptide was specifically designed to target and concentrate within the inner mitochondrial membrane, where it interacts with cardiolipin—a phospholipid essential for optimal mitochondrial function.
What makes SS-31 unique among peptides is its ability to cross cell membranes independently of mitochondrial membrane potential and accumulate at concentrations 1,000 to 5,000-fold higher within mitochondria compared to the cytosol. Unlike traditional antioxidants that neutralize reactive oxygen species (ROS) after they've formed, SS-31 works upstream by stabilizing the electron transport chain and preventing excessive ROS generation at its source.
The primary human-use benefits associated with SS-31 include enhanced ATP production, reduced oxidative damage, improved cardiac function, protection against ischemia-reperfusion injury, and potential applications in age-related mitochondrial decline. These properties have made it particularly appealing to those seeking to optimize cellular health and combat the bioenergetic deficits associated with aging and chronic disease.
How It Works
Cardiolipin Stabilization
The central mechanism of SS-31 involves its selective binding to cardiolipin, a unique phospholipid found almost exclusively in the inner mitochondrial membrane. Cardiolipin plays a critical role in organizing the electron transport chain complexes and maintaining cristae structure. With age and disease, cardiolipin becomes oxidized and depleted, leading to mitochondrial dysfunction.
SS-31 binds to cardiolipin through electrostatic and hydrophobic interactions, protecting it from oxidative damage and preserving its functional conformation. This stabilization helps maintain proper electron transport chain assembly and prevents cytochrome c peroxidase activity, which would otherwise generate harmful ROS.
Electron Transport Chain Optimization
By preserving cardiolipin integrity, SS-31 optimizes electron flow through complexes I, III, and IV of the electron transport chain. This results in more efficient oxidative phosphorylation, increased ATP synthesis, and reduced electron leak that would otherwise generate superoxide radicals. Studies have demonstrated that SS-31 can improve ATP production by 30–60% in dysfunctional mitochondria while simultaneously reducing ROS generation.
Mitochondrial Dynamics and Biogenesis
Emerging evidence suggests SS-31 may influence mitochondrial dynamics by promoting fusion over fission and supporting mitochondrial biogenesis through activation of PGC-1α signaling pathways. These effects contribute to healthier mitochondrial networks and improved cellular resilience against metabolic stress.
Anti-Inflammatory Effects
Beyond its direct mitochondrial actions, SS-31 demonstrates anti-inflammatory properties by reducing NLRP3 inflammasome activation and decreasing pro-inflammatory cytokine release. This occurs secondary to reduced mitochondrial ROS, which serves as a key trigger for inflammatory signaling cascades.
Dosage Protocols
Dosing protocols for SS-31 vary based on the condition being addressed and the route of administration. Clinical trials have employed a range of dosing strategies:
For subcutaneous administration, doses typically range from 10–40 mg once daily. The EMBRACE-STEMI trial utilized a single 0.05 mg/kg intravenous infusion, while heart failure studies have employed 4 mg/hour intravenous infusions over 4 hours for 5 consecutive days.
In the biohacking community, common protocols include 20–40 mg subcutaneously once daily for 4–8 weeks, followed by a maintenance phase of 2–3 times weekly. Some users implement cycling schedules of 8 weeks on, 4 weeks off, though the necessity of cycling remains unclear given the peptide's mechanism of action.
For acute applications such as exercise performance or recovery, single doses of 20–40 mg administered 1–2 hours before activity have been reported. Chronic protocols for age-related decline or mitochondrial support typically span 12–24 weeks at consistent daily dosing.
How to Use / Administration Methods
SS-31 is most commonly administered via subcutaneous injection, though intravenous infusion has been used in clinical settings. For subcutaneous use:
Reconstitute lyophilized SS-31 with bacteriostatic water to achieve the desired concentration (typically 5–10 mg/mL). Draw the appropriate dose using an insulin syringe. Inject subcutaneously into the abdominal area, rotating injection sites to prevent lipodystrophy. Morning administration is often preferred to align with natural circadian rhythms of mitochondrial function.
Intravenous administration, while more bioavailable, requires clinical supervision and is not practical for most users. Topical and oral formulations have been explored but demonstrate significantly reduced bioavailability compared to injectable routes.
Results Timelines
The timeline for experiencing benefits from SS-31 varies by application:
Acute effects (hours to days): Improved exercise tolerance, reduced post-exertion fatigue, and enhanced recovery may be noticed within the first few doses. Some users report increased mental clarity and energy within 1–2 hours of administration.
Short-term effects (1–4 weeks): Measurable improvements in cardiac function, exercise capacity, and biomarkers of oxidative stress have been documented in clinical trials within this timeframe. Users commonly report sustained energy improvements and better stress resilience.
Long-term effects (4–12+ weeks): Structural and functional improvements in mitochondrial health, including enhanced mitochondrial biogenesis and improved tissue function, develop over extended treatment periods. Clinical trials in Barth syndrome and primary mitochondrial myopathy have demonstrated progressive improvements over 12–36 weeks of treatment.
Research Evidence
SS-31 has been evaluated in numerous preclinical and clinical studies across various conditions:
The EMBRACE-STEMI trial examined SS-31 in patients with ST-elevation myocardial infarction undergoing percutaneous coronary intervention. While the primary endpoint of infarct size reduction was not met, subgroup analyses suggested potential benefits in anterior infarcts.
In heart failure with reduced ejection fraction, the PROGRESS-HF trial demonstrated that SS-31 improved left ventricular end-systolic volume and was associated with trends toward improved cardiac function, though primary endpoints were not achieved.
Studies in Barth syndrome, a genetic disorder characterized by cardiolipin deficiency, have shown promising results with improvements in 6-minute walk distance and cardiac function. The TAZPOWER trial demonstrated clinically meaningful improvements in functional capacity.
Preclinical research has demonstrated protective effects against age-related cardiac dysfunction, renal ischemia-reperfusion injury, skeletal muscle atrophy, and neurodegenerative processes. Animal studies consistently show improved mitochondrial function, reduced oxidative stress, and enhanced tissue protection across multiple organ systems.
Stacking
SS-31 may be combined with other compounds to enhance mitochondrial support:
With NAD+ precursors (NMN/NR): Combining SS-31 with nicotinamide mononucleotide or nicotinamide riboside may provide synergistic benefits by simultaneously optimizing electron transport chain function and NAD+ availability for mitochondrial enzymes.
With CoQ10/Ubiquinol: As CoQ10 serves as an electron carrier in the same pathway SS-31 optimizes, co-administration may enhance overall electron transport efficiency.
With PQQ: Pyrroloquinoline quinone supports mitochondrial biogenesis through different mechanisms, potentially complementing SS-31's protective effects.
With BPC-157: Some users combine SS-31 with BPC-157 for enhanced tissue healing and recovery applications.
Caution is advised when stacking, as interactions have not been formally studied. Starting with SS-31 alone before adding additional compounds allows for better assessment of individual response.
Reconstitution, Storage & Preparation
SS-31 is typically supplied as a lyophilized (freeze-dried) powder requiring reconstitution before use:
Store unreconstituted SS-31 at -20°C for long-term storage or 2–8°C for short-term storage (up to several months). Protect from light and moisture. For reconstitution, use bacteriostatic water for multi-dose vials. Add the diluent slowly to the vial wall, allowing the powder to dissolve without agitation. Gently swirl if needed—do not shake vigorously.
Once reconstituted, store at 2–8°C and use within 28 days. Some degradation may occur over time; discard if the solution becomes cloudy or discolored. For single-use applications, sterile water for injection may be used, but the vial should not be stored after initial use.
Side Effects
SS-31 has demonstrated a favorable safety profile in clinical trials, with most adverse events being mild and transient:
Common side effects: Injection site reactions (redness, swelling, mild pain), headache, and nausea have been reported in clinical trials at rates slightly higher than placebo.
Less common effects: Some participants have experienced dizziness, fatigue, or gastrointestinal discomfort. These effects typically resolve without intervention.
Serious adverse events: No serious adverse events have been definitively attributed to SS-31 in published clinical trials. However, long-term safety data in healthy populations remains limited.
Theoretical concerns: As SS-31 modifies fundamental cellular processes, theoretical concerns exist regarding effects on cellular signaling and potential interactions with cancer biology, though no evidence of carcinogenicity has emerged.
Legal Status / FDA
SS-31 (Elamipretide) is currently an investigational drug that has not received FDA approval for any indication. It has been granted Fast Track designation, Orphan Drug designation, and Rare Pediatric Disease designation for Barth syndrome and primary mitochondrial myopathy.
In the United States, SS-31 exists in a regulatory gray area when obtained through research chemical suppliers. It is not a controlled substance, but selling it for human consumption is prohibited. Possession for personal research purposes generally falls outside regulatory enforcement priorities.
International regulations vary significantly. Users should familiarize themselves with local laws regarding peptide possession and use.
Sports/WADA Status
SS-31 is not currently listed on the World Anti-Doping Agency (WADA) Prohibited List. However, its mechanism of action—enhancing mitochondrial function and potentially improving exercise capacity—could theoretically provide performance-enhancing benefits.
Athletes subject to anti-doping regulations should exercise caution, as WADA reserves the right to add substances to the prohibited list and may test for novel compounds. The peptide's potential to enhance oxygen utilization and ATP production could attract regulatory scrutiny as research progresses.
Conclusion
SS-31 represents a novel approach to addressing mitochondrial dysfunction through targeted cardiolipin stabilization and electron transport chain optimization. While clinical trial results have been mixed, the peptide demonstrates a favorable safety profile and consistent improvements in biomarkers of mitochondrial function. For individuals seeking to support cellular energy production and combat age-related bioenergetic decline, SS-31 offers a mechanistically unique option backed by substantial preclinical evidence and ongoing clinical investigation.
FAQ
What is the difference between SS-31 and Elamipretide?
They are the same compound. SS-31 is the research designation, while Elamipretide is the generic pharmaceutical name. Bendavia and MTP-131 are additional names used in various clinical trials.
How quickly does SS-31 work?
Acute effects on energy and exercise tolerance may be noticed within hours of administration. Measurable improvements in cardiac function and biomarkers typically emerge over 1–4 weeks, while structural mitochondrial improvements develop over months.
Can SS-31 be taken orally?
Oral bioavailability is significantly reduced compared to injectable administration. While oral formulations have been explored, subcutaneous injection remains the preferred route for optimal absorption.
Does SS-31 require cycling?
The necessity of cycling is not established. Clinical trials have used continuous dosing for up to 36 weeks without apparent tolerance development. Some users implement cycling protocols as a precautionary measure.
Is SS-31 safe for long-term use?
Clinical trials up to 36 weeks have not revealed significant safety concerns. However, long-term data in healthy populations is limited, and users should monitor for any adverse effects.
Can SS-31 be combined with other peptides?
While formal interaction studies are lacking, SS-31 has been theoretically combined with other mitochondrial support compounds. Starting with SS-31 alone before adding other substances is advisable.
What conditions might benefit from SS-31?
Research has focused on heart failure, ischemia-reperfusion injury, primary mitochondrial diseases, Barth syndrome, and age-related mitochondrial decline. Preclinical evidence suggests potential applications in renal, neurological, and skeletal muscle conditions.
How should SS-31 be stored?
Lyophilized powder should be stored at -20°C for long-term storage or 2–8°C for shorter periods. Reconstituted solution should be refrigerated and used within 28 days.
References
- Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol. 2014;171(8):2029-2050. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4076984/
- Birk AV, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol. 2013;24(8):1250-1261. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3770734/
- Daubert MA, et al. Novel Mitochondria-Targeting Peptide in Heart Failure Treatment: A Randomized, Placebo-Controlled Trial of Elamipretide. Circ Heart Fail. 2017;10(12):e004230. https://www.ahajournals.org/doi/10.1161/JAHA.116.004230
- Butler J, et al. Effects of Elamipretide on Left Ventricular Function in Patients With Heart Failure With Reduced Ejection Fraction: The PROGRESS-HF Phase 2 Trial. J Card Fail. 2020;26(5):429-437. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8554896/
- Thompson R, et al. Current and future treatment approaches for Barth syndrome. J Inherit Metab Dis. 2022;45(1):17-28. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8285433/
- Karaa A, et al. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology. 2018;90(14):e1212-e1221. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5890604/
- Stealth BioTherapeutics. Elamipretide Development Program. https://www.stealthbt.com/programs/elamipretide/
- Sabbah HN, et al. Chronic Therapy With Elamipretide (MTP-131), a Novel Mitochondria-Targeting Peptide, Improves Left Ventricular and Mitochondrial Function in Dogs With Advanced Heart Failure. Circ Heart Fail. 2016;9(2):e002206. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5500439/
- Gibson CM, et al. EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur Heart J. 2016;37(16):1296-1303. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6857653/