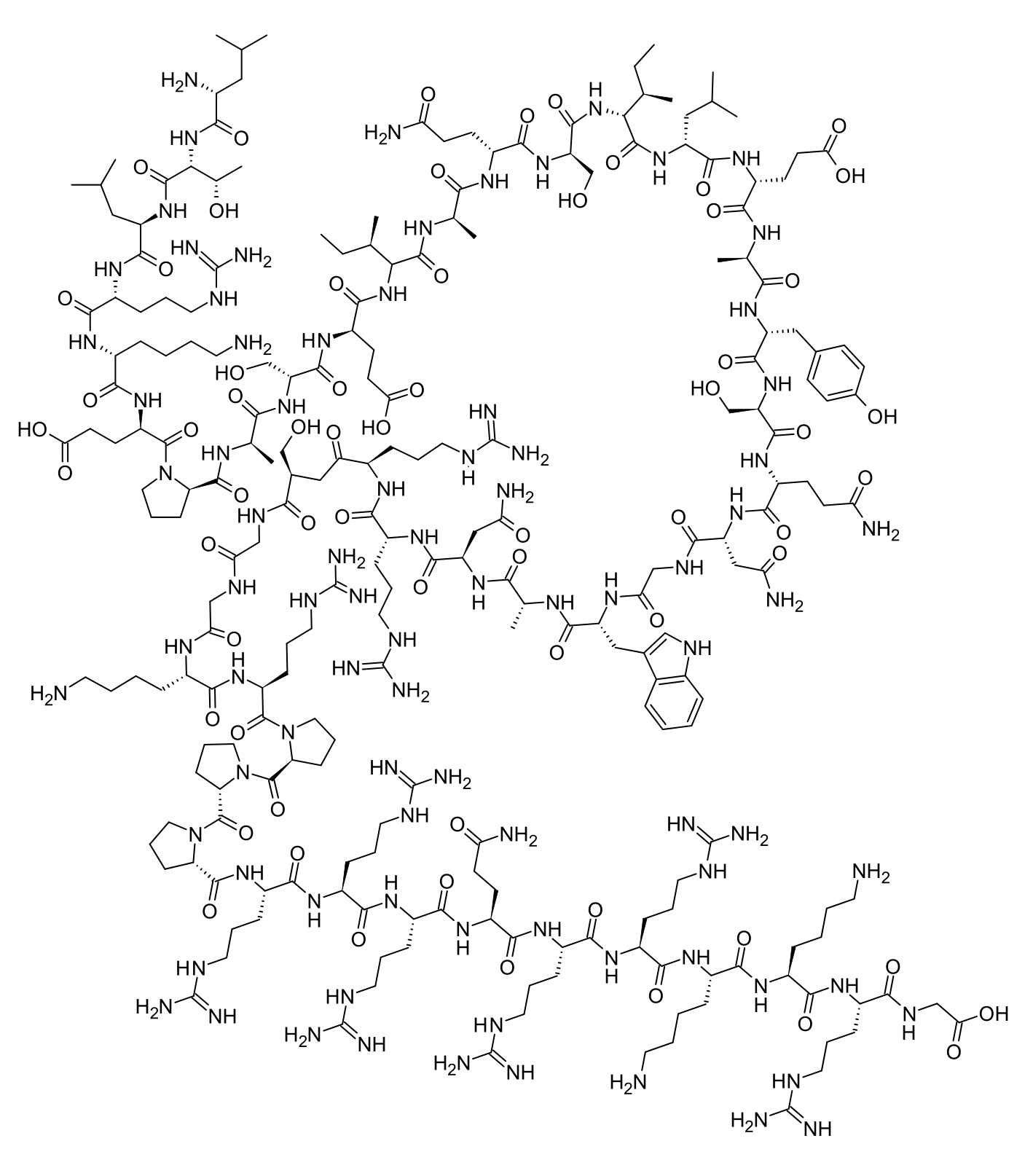
FOXO4-DRI
From Peptidepedia, the trusted peptide wiki.


- FOXO4-DRI is a synthetic peptide that eliminates aged, dysfunctional cells by disrupting protein interactions.
- The peptide works by releasing p53 protein, triggering programmed cell death in senescent cells only.
- Animal studies showed improved organ function and tissue regeneration, but human testing remains experimental.
FOXO4-DRI is a synthetic peptide designed to selectively eliminate senescent (aged, dysfunctional) cells by disrupting the interaction between FOXO4 and p53 proteins, triggering apoptosis in these "zombie cells" while sparing healthy tissue. Popular among longevity researchers, biohackers, and anti-aging enthusiasts, FOXO4-DRI has garnered attention for its potential to reverse age-related tissue deterioration, improve organ function, and extend healthspan. Typical dosing protocols range from 2-5 mg/kg administered subcutaneously, often in cycles of 3 consecutive days repeated monthly for 2-3 months, though human dosing remains experimental and extrapolated from animal studies.
What Is FOXO4-DRI?
FOXO4-DRI (FOXO4-D-Retro-Inverso) is a modified peptide consisting of 46 amino acids arranged in a D-retro-inverso configuration, meaning the peptide sequence is reversed and uses D-amino acids instead of the naturally occurring L-amino acids. This structural modification significantly enhances the peptide's stability and resistance to enzymatic degradation while maintaining its biological activity.
The peptide was developed by researchers at Erasmus University Medical Center in the Netherlands, led by Dr. Peter de Keizer, and first published in the journal Cell in March 2017. The groundbreaking research demonstrated that FOXO4-DRI could selectively induce apoptosis in senescent cells, effectively clearing them from tissues and restoring organ function in aged mice.
What makes FOXO4-DRI unique in the senolytic landscape is its targeted mechanism. Unlike broad-spectrum senolytics such as dasatinib and quercetin, which affect multiple cellular pathways, FOXO4-DRI specifically disrupts a single protein-protein interaction that senescent cells depend upon for survival. This precision theoretically reduces off-target effects while maximizing senescent cell clearance.
Primary human-use benefits attributed to FOXO4-DRI include potential reversal of age-related physical decline, improved kidney function, enhanced fur density and coat quality (observed in animal models), increased vitality, and possible extension of healthspan. Users report improvements in skin quality, energy levels, recovery from exercise, and overall sense of well-being, though these remain anecdotal observations.
How It Works
The Senescence-Survival Mechanism
Cellular senescence is a state where cells permanently stop dividing but resist apoptosis (programmed cell death). These senescent cells accumulate with age and secrete inflammatory factors known as the senescence-associated secretory phenotype (SASP), which damages surrounding healthy tissue and contributes to aging and age-related diseases.
Senescent cells survive by upregulating specific survival pathways. One critical mechanism involves the FOXO4 protein binding to p53, a tumor suppressor protein often called the "guardian of the genome." When FOXO4 sequesters p53 in the nucleus, it prevents p53 from triggering apoptosis, allowing the senescent cell to persist indefinitely.
FOXO4-p53 Disruption
FOXO4-DRI works by competitively binding to p53, displacing the endogenous FOXO4 protein. The D-retro-inverso configuration allows the peptide to mimic the binding interface of natural FOXO4 while being resistant to proteolytic degradation. Once FOXO4-DRI occupies the p53 binding site, the natural FOXO4 can no longer maintain its protective interaction.
With FOXO4 displaced, p53 is released from nuclear sequestration and can relocate to the mitochondria, where it initiates the intrinsic apoptotic pathway. This triggers cytochrome c release, caspase activation, and ultimately programmed cell death of the senescent cell.
Selectivity for Senescent Cells
The selectivity of FOXO4-DRI stems from the fact that healthy, non-senescent cells do not rely on the FOXO4-p53 interaction for survival. In normal cells, p53 levels are tightly regulated and typically low, and the FOXO4-p53 binding is not a critical survival mechanism. Senescent cells, however, have elevated p53 levels and depend heavily on FOXO4 to neutralize p53's pro-apoptotic functions.
This differential dependency creates a therapeutic window where FOXO4-DRI can eliminate senescent cells while leaving healthy cells unaffected. The original research demonstrated this selectivity both in vitro and in vivo, with treated mice showing senescent cell clearance without apparent toxicity to normal tissues.
Downstream Effects of Senescent Cell Clearance
When senescent cells are eliminated, the local tissue environment improves dramatically. The inflammatory SASP factors decrease, allowing tissue regeneration and reducing chronic low-grade inflammation (inflammaging). In the 2017 study, aged mice treated with FOXO4-DRI showed restored fitness, improved kidney function, and regrowth of fur—markers of genuine biological rejuvenation rather than mere symptom suppression.
Dosage Protocols
Human dosing for FOXO4-DRI remains experimental, as no clinical trials have established optimal protocols. Current recommendations are extrapolated from the original mouse studies and adjusted based on user reports from the biohacking community.
The mouse studies used approximately 5 mg/kg body weight administered intravenously. For human subcutaneous administration, commonly reported protocols include 2-5 mg/kg, with most users starting at the lower end.
A typical cycling protocol involves:
- 3 consecutive days of administration
- 2-4 week rest period
- Repeat for 2-3 cycles
- Reassess and potentially repeat after 3-6 months
Some users prefer a modified protocol of once-weekly injections for 4-6 weeks, followed by an extended break. The rationale for cycling is to allow the body time to clear dead senescent cells and regenerate tissue between treatment periods.
Given the peptide's experimental status, conservative dosing with careful self-monitoring is strongly advised. Starting with lower doses and gradually increasing allows assessment of individual tolerance.
How to Use / Administration Methods
FOXO4-DRI is typically administered via subcutaneous injection, the most practical method for self-administration. The peptide is supplied as a lyophilized (freeze-dried) powder that requires reconstitution before use.
For subcutaneous injection:
- Reconstitute the peptide with bacteriostatic water
- Draw the calculated dose into an insulin syringe
- Inject into subcutaneous fat (abdomen, thigh, or upper arm)
- Rotate injection sites to prevent tissue irritation
Some researchers have explored intravenous administration, which was used in the original studies, but this method carries additional risks and is not recommended for non-clinical settings.
The peptide should be injected on an empty stomach or at least 2 hours after eating, as some users report improved absorption under fasting conditions.
Results Timelines
Based on animal research and user reports, the timeline for observable effects varies:
Weeks 1-2: Most users report no immediately noticeable changes. Cellular processes of senescent cell clearance and debris removal are occurring at the microscopic level.
Weeks 3-4: Some users begin reporting subtle improvements in energy levels, sleep quality, and general well-being. Skin texture improvements may become apparent.
Weeks 6-8: More pronounced effects may emerge, including improved exercise recovery, enhanced cognitive clarity, and visible improvements in skin and hair quality.
Months 2-3: The full benefits of senescent cell clearance typically manifest, with users reporting sustained improvements in physical function, reduced joint discomfort, and overall vitality.
It's important to note that results are highly individual and depend on baseline senescent cell burden, age, overall health status, and lifestyle factors.
Research Evidence
The foundational research for FOXO4-DRI was published in Cell in 2017 by de Keizer and colleagues. This study demonstrated that FOXO4-DRI could neutralize senescent cells in vitro and restore health parameters in fast-aging and naturally aged mice.
Key findings from the original research included:
- Selective induction of apoptosis in senescent cells
- Restoration of fur density in aged mice
- Improved kidney function markers
- Enhanced physical fitness and activity levels
- No apparent toxicity to healthy tissues
Subsequent research has explored the broader field of senolytics, with studies confirming that senescent cell clearance can extend healthspan and delay age-related pathology in various animal models.
However, it must be emphasized that no human clinical trials of FOXO4-DRI have been published. All human use remains experimental and based on extrapolation from preclinical data.
Stacking
Some users combine FOXO4-DRI with other senolytic or longevity-focused compounds:
Dasatinib + Quercetin (D+Q): This combination targets different senescent cell populations and may provide complementary clearance. D+Q is typically used on alternate cycles rather than simultaneously with FOXO4-DRI.
Fisetin: A natural flavonoid with senolytic properties that some users add to their protocol for broader coverage.
NAD+ precursors (NMN/NR): These compounds support cellular energy metabolism and may enhance tissue regeneration following senescent cell clearance.
Rapamycin: Some longevity protocols combine mTOR inhibition with senolytic therapy, though this requires careful consideration of immune effects.
Stacking should be approached cautiously, as interactions between experimental compounds are poorly characterized.
Reconstitution, Storage & Prep
FOXO4-DRI is supplied as a lyophilized powder and requires proper handling:
Reconstitution:
- Use bacteriostatic water (BAC water) for reconstitution
- Add water slowly, directing the stream against the vial wall
- Gently swirl—never shake—until fully dissolved
- Allow any foam to settle before drawing doses
Storage:
- Lyophilized powder: Store at -20°C for long-term stability; refrigeration (2-8°C) acceptable for shorter periods
- Reconstituted solution: Refrigerate at 2-8°C and use within 3-4 weeks
- Protect from light and temperature fluctuations
- Never freeze reconstituted peptide
Preparation tips:
- Calculate doses carefully based on reconstitution volume
- Use insulin syringes for accurate measurement
- Maintain sterile technique throughout handling
Side Effects
Reported side effects from user experiences include:
Common:
- Injection site reactions (redness, mild swelling)
- Transient flu-like symptoms (possibly from senescent cell die-off)
- Fatigue during initial treatment days
- Mild headache
Less common:
- Gastrointestinal discomfort
- Temporary increase in inflammatory markers
- Dizziness
Theoretical concerns:
- Unknown long-term effects of repeated senescent cell clearance
- Potential impact on beneficial senescent cells (wound healing, tumor suppression)
- Possible immune system effects
The original mouse studies did not report significant adverse effects, but the absence of human clinical data means the full safety profile remains unknown.
Legal Status / FDA
FOXO4-DRI is not approved by the FDA or any regulatory agency for human use. It is classified as a research chemical and sold for laboratory research purposes only. Possession and personal use exist in a legal gray area in most jurisdictions, similar to other research peptides.
In the United States, purchasing FOXO4-DRI for personal research is generally not prohibited, but selling it for human consumption would violate FDA regulations. Users should understand they are assuming full responsibility for any self-experimentation.
Sports / WADA Status
FOXO4-DRI is not specifically listed on the World Anti-Doping Agency (WADA) Prohibited List. However, WADA's regulations include provisions against substances that are not approved for human therapeutic use, and peptides with performance-enhancing potential may fall under the S0 category (non-approved substances).
Athletes subject to anti-doping regulations should assume that FOXO4-DRI use could result in sanctions and should consult with relevant authorities before considering use.
Conclusion
FOXO4-DRI represents a fascinating advancement in senolytic research, offering a targeted approach to eliminating the senescent cells that contribute to aging and age-related disease. The peptide's mechanism—disrupting the FOXO4-p53 interaction to selectively trigger apoptosis in senescent cells—provides a level of precision not seen with other senolytic approaches.
While the preclinical evidence is compelling, the absence of human clinical trials means that all current use is experimental. Those choosing to explore FOXO4-DRI should do so with full awareness of the unknown risks, careful attention to dosing and administration, and realistic expectations about outcomes.
As the field of senolytics continues to evolve, FOXO4-DRI remains one of the most intriguing candidates for translating the promise of senescent cell clearance into tangible human benefits.
FAQ
What is the difference between FOXO4-DRI and other senolytics like dasatinib and quercetin?
FOXO4-DRI targets a specific protein-protein interaction (FOXO4-p53) that senescent cells depend on for survival, while dasatinib and quercetin work through broader mechanisms affecting multiple pathways. This specificity may reduce off-target effects but might also mean different senescent cell populations are targeted.
How long does FOXO4-DRI remain stable after reconstitution?
When properly stored at 2-8°C and protected from light, reconstituted FOXO4-DRI typically remains stable for 3-4 weeks. Using bacteriostatic water rather than sterile water extends stability by preventing bacterial growth.
Can FOXO4-DRI be used alongside other anti-aging interventions?
Many users combine FOXO4-DRI with other longevity interventions, though interactions are not well-characterized. Common combinations include NAD+ precursors and other senolytics used on alternating schedules.
What age is appropriate to begin FOXO4-DRI use?
Senescent cell accumulation increases significantly after age 40-50. Most users are in this age range or older, though some younger individuals with accelerated aging markers have explored its use.
How do I know if FOXO4-DRI is working?
Objective markers are difficult to assess without laboratory testing. Subjective improvements in energy, skin quality, recovery, and overall vitality are commonly reported indicators, though these are not definitive.
Is FOXO4-DRI the same as FOXO4?
No. FOXO4 is a naturally occurring protein in the body. FOXO4-DRI is a synthetic peptide designed to interfere with FOXO4's function specifically in senescent cells.
What happens to the dead senescent cells after treatment?
Dead cells are cleared by the immune system through phagocytosis. This process may contribute to temporary flu-like symptoms as cellular debris is processed and eliminated.
Are there any contraindications for FOXO4-DRI use?
Given the lack of clinical data, specific contraindications are not established. Theoretical concerns include active infections, autoimmune conditions, recent surgery, pregnancy, and cancer, where senescent cells may play complex roles.
References
- Baar MP, et al. "Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging." Cell. 2017;169(1):132-147. https://www.cell.com/cell/fulltext/S0092-8674(17)30246-5
- Di Micco R, et al. "Cellular senescence in ageing: from mechanisms to therapeutic opportunities." Nature Reviews Molecular Cell Biology. 2021;22:75-95. https://www.nature.com/articles/s41580-020-00314-w
- Kirkland JL, Tchkonia T. "Senolytic drugs: from discovery to translation." Journal of Internal Medicine. 2020;288(5):518-536. https://onlinelibrary.wiley.com/doi/10.1111/joim.13141
- Xu M, et al. "Senolytics improve physical function and increase lifespan in old age." Nature Medicine. 2018;24:1246-1256. https://www.nature.com/articles/s41591-018-0092-9
- Childs BG, et al. "Senescent cells: an emerging target for diseases of ageing." Nature Reviews Drug Discovery. 2017;16:718-735. https://www.nature.com/articles/nrd.2017.116
- World Anti-Doping Agency. "The Prohibited List." https://www.wada-ama.org/en/prohibited-list
- Robbins PD, et al. "Senolytic Drugs: Reducing Senescent Cell Viability to Extend Health Span." Annual Review of Pharmacology and Toxicology. 2021;61:779-803. https://www.annualreviews.org/doi/10.1146/annurev-pharmtox-050120-105018
- Zhu Y, et al. "The Achilles' heel of senescent cells: from transcriptome to senolytic drugs." Aging Cell. 2015;14(4):644-658. https://onlinelibrary.wiley.com/doi/10.1111/acel.12344