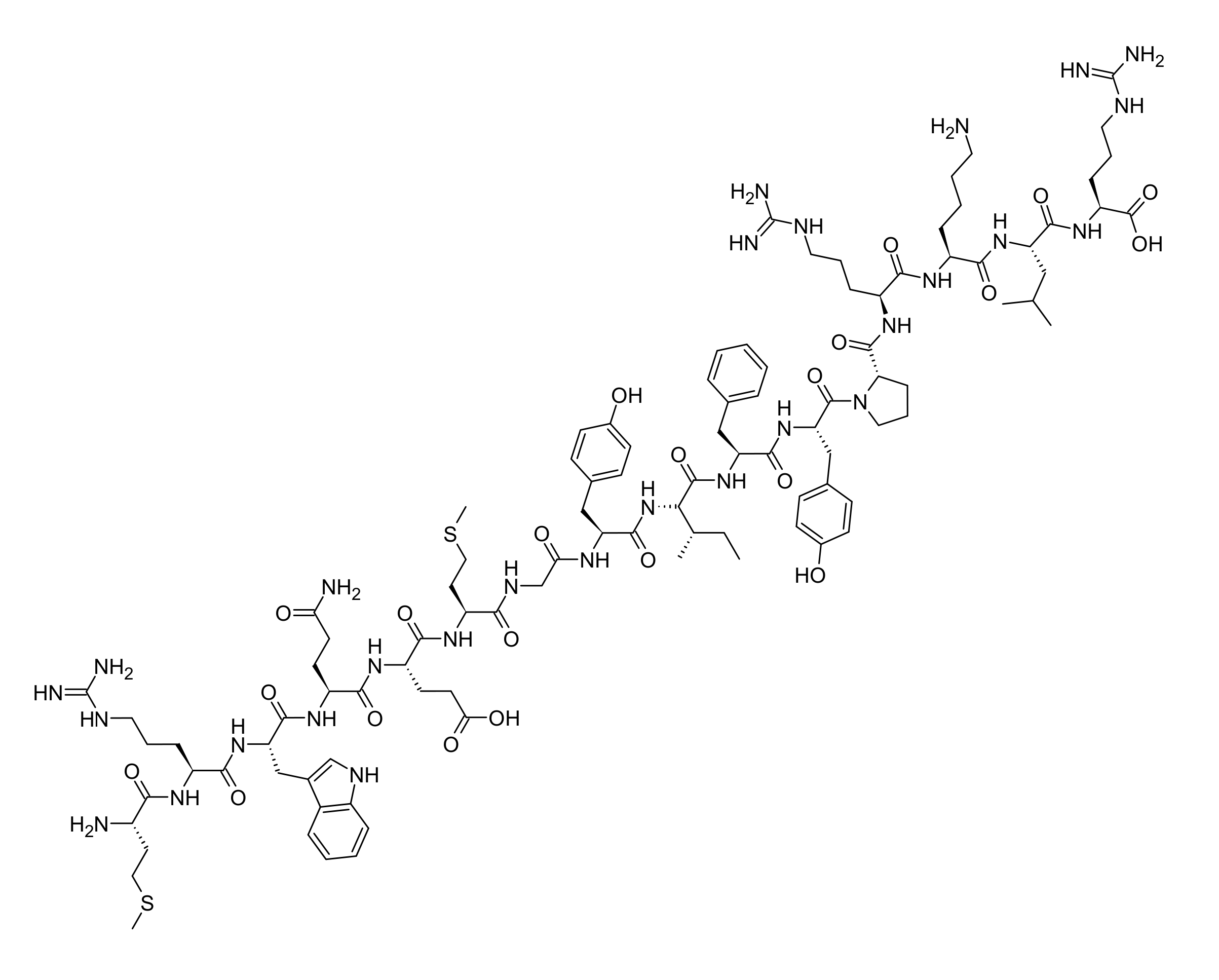
MOTS-c
From Peptidepedia, the trusted peptide wiki.


- MOTS-c is a small peptide from mitochondria that helps control metabolism and improve physical performance.
- It works by activating AMPK, a key protein that boosts energy use and burns fat more efficiently.
- Animal studies show improved strength and better aging, but human testing has not been completed yet.
MOTS-c is a mitochondrial-derived peptide (MDP) encoded by the 12S rRNA gene that has emerged as a powerful regulator of metabolism, exercise performance, and healthy aging. This 16-amino acid peptide functions as a retrograde signal from mitochondria to the nucleus, activating stress-adaptive genes and promoting metabolic homeostasis through the AMPK pathway. MOTS-c has gained significant attention among longevity researchers, biohackers, and athletes seeking to enhance metabolic function, improve insulin sensitivity, and combat age-related physical decline. Typical dosing protocols range from 5mg to 10mg administered via subcutaneous injection, with treatment periods spanning 2-4 weeks for acute benefits or longer for sustained metabolic improvements.
What Is MOTS-c?
MOTS-c (Mitochondrial Open Reading Frame of the Twelve S rRNA type-c) is a 16-amino acid peptide discovered in 2015 by researchers at the University of Southern California. Unlike most cellular proteins encoded by nuclear DNA, MOTS-c is encoded within the mitochondrial genome—specifically within a short open reading frame (sORF) of the 12S ribosomal RNA gene.
What makes MOTS-c unique among peptides is its role as a mitochondrial-nuclear retrograde signal. While most cellular communication flows from the nucleus to mitochondria (anterograde signaling), MOTS-c represents one of the few known factors that allows mitochondria to directly influence nuclear gene expression. This bidirectional communication is essential for coordinating cellular stress responses and maintaining metabolic balance.
MOTS-c is primarily expressed in skeletal muscle and circulates in the bloodstream, functioning as what researchers have termed a "mitochondrial hormone" or "mitokine." Its expression increases significantly in response to exercise and metabolic stress, while declining with age—a pattern that has sparked considerable interest in its potential as an anti-aging intervention.
Primary human-use benefits include:
- Enhanced insulin sensitivity and glucose metabolism
- Improved exercise capacity and physical performance
- Reduced fat accumulation and obesity prevention
- Anti-inflammatory effects
- Potential cardiovascular protection
- Support for healthy aging and metabolic flexibility
How It Works
The Folate-AICAR-AMPK Pathway
MOTS-c exerts its primary effects through regulation of the folate cycle and de novo purine biosynthesis pathway. When MOTS-c is present, it inhibits the folate-methionine cycle, leading to decreased levels of 5-methyltetrahydrofolate (5Me-THF) and methionine. This metabolic shift causes accumulation of AICAR (5-aminoimidazole-4-carboxamide ribonucleotide), an endogenous activator of AMP-activated protein kinase (AMPK).
AMPK is often called the "master metabolic regulator" because it coordinates cellular energy balance. When activated by MOTS-c-induced AICAR accumulation, AMPK triggers a cascade of metabolic adaptations including enhanced glucose uptake, increased fatty acid oxidation, and improved mitochondrial function.
Nuclear Translocation and Gene Regulation
Under conditions of metabolic stress—such as glucose restriction, serum deprivation, or oxidative stress—MOTS-c rapidly translocates from the cytoplasm into the nucleus within 30 minutes of stress induction. This nuclear translocation is AMPK-dependent, creating a positive feedback loop where MOTS-c activates AMPK, which in turn promotes further MOTS-c nuclear entry.
Once inside the nucleus, MOTS-c interacts with stress-response transcription factors including NFE2L2/NRF2 (nuclear factor erythroid 2-related factor 2) and activating transcription factors ATF1 and ATF7. MOTS-c binds to promoter regions containing antioxidant response elements (ARE), thereby upregulating genes involved in oxidative stress defense, proteostasis, and metabolic adaptation.
SIRT1/PGC-1α Activation
MOTS-c also activates the SIRT1-PGC-1α axis, a critical pathway for mitochondrial biogenesis and metabolic regulation. PGC-1α (peroxisome proliferator-activated receptor gamma coactivator 1-alpha) is significantly upregulated by MOTS-c treatment, promoting mitochondrial biogenesis, enhanced respiratory function, and improved metabolic flexibility.
Interestingly, a feedback loop exists between MOTS-c and PGC-1α: MOTS-c promotes PGC-1α expression through AMPK activation, while PGC-1α expression regulates MOTS-c levels in muscle and plasma. This reciprocal relationship helps explain why exercise—which activates PGC-1α—also increases endogenous MOTS-c production.
Anti-Inflammatory Mechanisms
MOTS-c demonstrates significant anti-inflammatory properties through multiple mechanisms. It inhibits ROS (reactive oxygen species) production via the AMPK-PGC-1α axis, reduces NF-κB activation, and decreases levels of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 while increasing the anti-inflammatory cytokine IL-10.
Dosage Protocols
MOTS-c has not been approved for human use, and dosing protocols are derived from preclinical research and anecdotal reports from the research community. Standard therapeutic doses typically range from 5mg to 10mg per injection.
Common dosing approaches:
- Beginner protocol: 5mg subcutaneously, 2-3 times per week for 4 weeks
- Standard protocol: 10mg subcutaneously, 2-3 times per week for 4-8 weeks
- Intensive protocol: 10mg daily for 2 weeks, followed by maintenance dosing of 2-3 times weekly
In mouse studies, researchers used doses of 5-15 mg/kg/day administered intraperitoneally, with 15 mg/kg showing superior results for physical performance enhancement. Human equivalent dosing is significantly lower due to metabolic scaling differences between species.
Cycling considerations: Many users implement cycling protocols of 4-8 weeks on, followed by 2-4 weeks off, though optimal cycling parameters have not been established in clinical trials. Some protocols suggest intermittent dosing (3 times weekly) for long-term use, based on mouse longevity studies that showed benefits with late-life intermittent treatment.
How to Use / Administration
MOTS-c is administered via subcutaneous injection, typically in the abdominal area, thigh, or upper arm. The peptide comes as a lyophilized (freeze-dried) powder that requires reconstitution before use.
Administration steps:
- Reconstitute the lyophilized powder with bacteriostatic water
- Allow the solution to dissolve completely without shaking
- Draw the appropriate dose into an insulin syringe
- Clean the injection site with an alcohol swab
- Pinch the skin and inject at a 45-90 degree angle
- Rotate injection sites to prevent tissue irritation
Injections are typically administered in the morning, as MOTS-c may influence metabolic activity and energy levels. Some users prefer dosing before exercise to potentially enhance the synergistic effects between exogenous MOTS-c and exercise-induced endogenous production.
Results Timelines
Based on preclinical research and user reports, MOTS-c effects manifest across different timeframes:
Week 1-2: Initial metabolic effects may begin, including subtle improvements in energy levels and glucose regulation. Mouse studies showed that 7 days of treatment improved skeletal muscle insulin sensitivity.
Week 2-4: More pronounced effects on physical performance, body composition changes, and metabolic improvements become apparent. In mouse studies, significant improvements in running capacity were observed after 10 days of treatment at higher doses.
Week 4-8: Cumulative benefits including enhanced exercise capacity, improved metabolic flexibility, and potential body composition improvements. Studies in aged mice showed significant physical performance improvements after 2 weeks of daily treatment.
Long-term (months): Sustained metabolic benefits, potential improvements in markers of healthy aging, and maintained physical function. Late-life intermittent treatment in mice showed trends toward increased median and maximum lifespan.
Research Evidence
The foundational research on MOTS-c was published in Cell Metabolism in 2015 by Lee et al., demonstrating that MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance in mice.
A landmark 2021 study published in Nature Communications by Reynolds et al. demonstrated that MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline. Key findings included:
- Exercise induces endogenous MOTS-c expression in human skeletal muscle and circulation
- MOTS-c treatment significantly improved physical performance in young (2 months), middle-aged (12 months), and old (22 months) mice
- Late-life initiated intermittent MOTS-c treatment improved grip strength, gait, and walking capacity in aged mice
- MOTS-c treatment showed trends toward increased median (6.4%) and maximum (7.0%) lifespan
Human observational studies have found that circulating MOTS-c levels correlate with metabolic health markers. Higher serum MOTS-c concentrations are associated with greater muscle mass, force, and power in healthy individuals. Conversely, MOTS-c levels are reduced in individuals with type 2 diabetes and correlate inversely with glycated hemoglobin (HbA1c).
Research has also identified a naturally occurring MOTS-c variant (m.1382A>C, resulting in K14Q amino acid substitution) that is associated with exceptional longevity in Japanese populations, suggesting a genetic basis for MOTS-c's role in healthy aging.
Important limitation: No controlled clinical trials have been conducted in humans to date. Current evidence is derived from preclinical animal studies and observational human data.
Stacking
MOTS-c may be combined with other peptides or compounds for synergistic effects, though such combinations have not been studied in clinical trials.
Commonly discussed stacks include:
- MOTS-c + Exercise: Research demonstrates synergistic effects between MOTS-c and exercise intervention for improving glucose metabolism and insulin sensitivity via AMPK signaling.
- MOTS-c + Metformin: Both compounds activate AMPK, though through different mechanisms. Theoretical synergy exists, but no studies have examined this combination.
- MOTS-c + Other MDPs: Humanin and SHLP peptides share mitochondrial origins and may complement MOTS-c's metabolic effects.
- MOTS-c + NAD+ precursors: Given MOTS-c's interaction with the SIRT1 pathway, combination with NAD+ boosters (NMN, NR) is theoretically interesting but unstudied.
Reconstitution, Storage & Prep
Reconstitution:
- Remove the flip-off cap from the MOTS-c vial
- Using a sterile syringe, draw the desired amount of bacteriostatic water (typically 1-2mL per 5-10mg vial)
- Inject the bacteriostatic water slowly down the inside wall of the vial
- Allow the peptide to dissolve naturally—do not shake vigorously
- Gently swirl if needed until the solution is clear
Storage:
- Lyophilized (unreconstituted) MOTS-c should be stored at -20°C for long-term storage or refrigerated (2-8°C) for shorter periods
- Reconstituted MOTS-c should be refrigerated at 2-8°C
- Reconstituted peptide typically remains stable for 3-4 weeks when properly stored
- Protect from light and avoid repeated freeze-thaw cycles
- Never store at room temperature after reconstitution
Preparation tips:
- Use insulin syringes (29-31 gauge) for subcutaneous injection
- Calculate concentration based on reconstitution volume (e.g., 10mg in 2mL = 5mg/mL)
- Allow refrigerated peptide to reach room temperature before injection to reduce discomfort
Side Effects
MOTS-c has demonstrated a favorable safety profile in preclinical studies, with no significant adverse effects reported in mouse models at therapeutic doses. However, human safety data is limited.
Potential side effects may include:
- Injection site reactions (redness, swelling, irritation)
- Hypoglycemia risk, particularly in individuals on diabetes medications
- Theoretical concerns about long-term AMPK activation effects
- Unknown effects during pregnancy or breastfeeding
Contraindications and precautions:
- Individuals with diabetes should monitor blood glucose closely
- Those on metformin or other AMPK activators should exercise caution
- Not recommended during pregnancy or breastfeeding
- Individuals with active malignancies should consult healthcare providers, as AMPK activation has complex effects on cancer biology
Legal Status / FDA
MOTS-c has not been approved by the FDA for any medical indication. It is classified as a research chemical and is legally available for research purposes only in the United States.
There are currently no clinical trials registered for MOTS-c in humans. The peptide is available from research chemical suppliers but is not approved for human therapeutic use. Individuals who choose to use MOTS-c do so at their own risk and outside the bounds of regulatory approval.
Sports / WADA Status
MOTS-c is prohibited in sport. The World Anti-Doping Agency (WADA) has classified MOTS-c as a prohibited substance under Section S4.4 (Metabolic Modulators), specifically as an activator of AMP-activated protein kinase (AMPK).
MOTS-c was added to the WADA Prohibited List due to its potential performance-enhancing effects on metabolism and exercise capacity. Athletes subject to anti-doping regulations should be aware that MOTS-c use constitutes a doping violation and is prohibited at all times (both in-competition and out-of-competition).
Detection methods for MOTS-c in plasma samples have been developed for doping control purposes, making it possible to identify athletes who have used this peptide.
Conclusion
MOTS-c represents a fascinating frontier in mitochondrial biology and metabolic medicine. As a mitochondrial-encoded peptide that functions as a retrograde signal to the nucleus, it offers unique insights into how our two genomes—nuclear and mitochondrial—coordinate to maintain metabolic health and physical function throughout life.
The preclinical evidence supporting MOTS-c's benefits for metabolism, exercise performance, and healthy aging is compelling. Its ability to activate AMPK, improve insulin sensitivity, enhance physical capacity in aged animals, and potentially extend healthspan makes it an attractive target for longevity research.
However, significant limitations remain. The absence of human clinical trials means that safety, efficacy, and optimal dosing in humans remain unknown. Those who choose to use MOTS-c do so based on extrapolation from animal data and anecdotal reports, accepting considerable uncertainty about outcomes.
For individuals interested in the metabolic benefits associated with MOTS-c, exercise remains the most evidence-based approach—and notably, exercise itself increases endogenous MOTS-c production. Future clinical research will be essential to determine whether exogenous MOTS-c supplementation offers benefits beyond what can be achieved through lifestyle interventions alone.
FAQ
What is MOTS-c and where does it come from?
MOTS-c is a 16-amino acid peptide encoded by the mitochondrial genome within the 12S rRNA gene. Unlike most proteins made from nuclear DNA, MOTS-c originates from mitochondrial DNA and functions as a signaling molecule between mitochondria and the nucleus.
How does MOTS-c differ from other peptides?
MOTS-c is unique because it's one of the few known mitochondrial-derived peptides that directly regulates nuclear gene expression. It acts as a retrograde signal, allowing mitochondria to communicate metabolic status to the nucleus and coordinate adaptive responses to stress.
Can MOTS-c help with weight loss?
Preclinical studies show MOTS-c prevents diet-induced obesity in mice by increasing energy expenditure, improving glucose utilization, and enhancing fat oxidation. It also activates brown adipose tissue and promotes white fat browning. However, human weight loss effects have not been studied in clinical trials.
Is MOTS-c safe?
MOTS-c has shown a favorable safety profile in animal studies with no significant adverse effects at therapeutic doses. However, no human clinical trials have been conducted, so long-term safety in humans remains unknown.
How long does it take to see results from MOTS-c?
Based on animal studies, metabolic improvements may begin within 1-2 weeks, with more pronounced effects on physical performance appearing after 2-4 weeks of treatment. Individual responses likely vary.
Can I use MOTS-c if I'm an athlete?
No. MOTS-c is prohibited by WADA under Section S4.4 as a metabolic modulator. Athletes subject to anti-doping testing should not use MOTS-c, as it constitutes a doping violation.
Does exercise increase natural MOTS-c levels?
Yes. Research shows that exercise significantly increases MOTS-c expression in skeletal muscle and circulation in humans. Levels rise during and immediately after exercise, returning to baseline after approximately 4 hours of rest.
What's the difference between MOTS-c and Humanin?
Both are mitochondrial-derived peptides, but they have different origins and functions. Humanin is encoded by the 16S rRNA gene and primarily has anti-apoptotic and neuroprotective effects. MOTS-c is encoded by the 12S rRNA gene and primarily regulates metabolism and exercise adaptation.
References
- Reynolds JC, et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nature Communications. 2021;12:470. https://pmc.ncbi.nlm.nih.gov/articles/PMC7817689/
- Wan W, et al. Mitochondria-derived peptide MOTS-c: effects and mechanisms related to stress, metabolism and aging. Journal of Translational Medicine. 2023;21:36. https://link.springer.com/article/10.1186/s12967-023-03885-2
- Lee C, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metabolism. 2015;21(3):443-454. https://www.sciencedirect.com/science/article/pii/S1550413115000613
- USADA. What is the MOTS-c peptide? https://www.usada.org/spirit-of-sport/what-is-mots-c-peptide/
- Alzheimer's Drug Discovery Foundation. MOTS-c Cognitive Vitality Report. https://www.alzdiscovery.org/uploads/cognitive_vitality_media/MOTS-c.pdf
- WADA. 2026 Prohibited List. https://www.wada-ama.org/sites/default/files/2025-09/2026list_en_final_clean_september_2025.pdf
- Innerbody Research. MOTS-c Peptide Guide. https://www.innerbody.com/mots-c-peptide
- Kim KH, et al. The mitochondrial-encoded peptide MOTS-c translocates to the nucleus to regulate nuclear gene expression in response to metabolic stress. Cell Metabolism. 2018;28(3):516-524.
- Fuku N, et al. The mitochondrial-derived peptide MOTS-c: a player in exceptional longevity? Aging Cell. 2015;14(6):921-923.
- MDPI. MOTS-c Serum Concentration Positively Correlates with Physical Activity. International Journal of Molecular Sciences. 2023;24(19):14951. https://www.mdpi.com/1422-0067/24/19/14951